Our goal is to combine advanced genomic and proteomic tools with innovative experimental models to study the regulation of the immune response in health and disease. In order to achieve this goal, our group is organized around the following research programs.
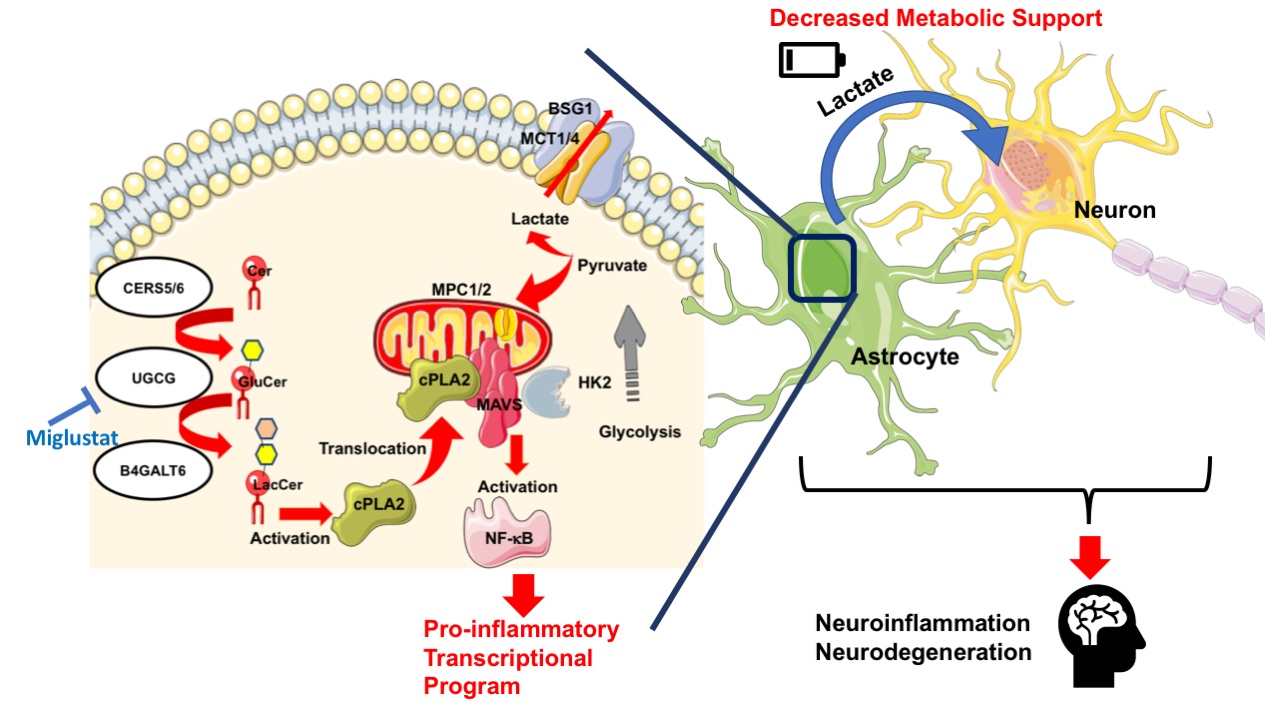
Sphingolipid metabolism in astrocytes activates the mitochondrial antiviral signaling protein (MAVS) through a mechanism mediated by cytosolic phospholipase A2 (cPLA2) to enhance NF-kB controlled neuroinflammation and to interfere with HK2-mediated metabolic support to neurons. Miglustat, an FDA approved drug, could potentially be applied for control of this immunological disorder.
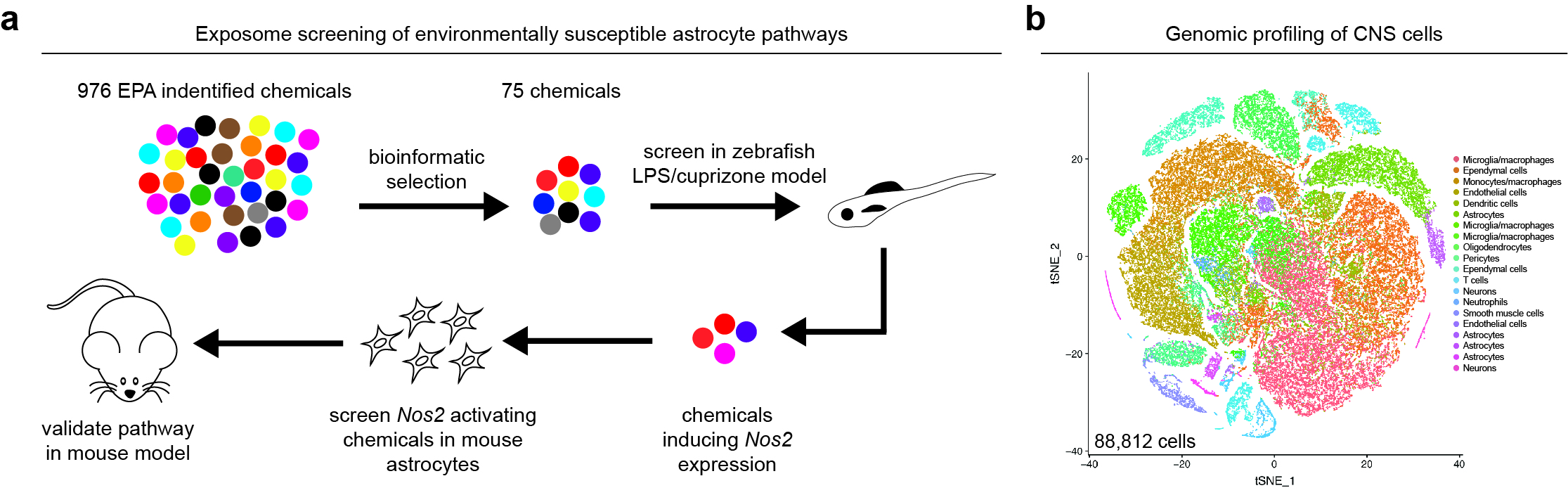
Regulation of astrocytes in the context of neurologic diseases, specifically those that involve CNS inflammation. To identify candidate regulators of astrocyte pathogenic activities as we have developed novel screening platforms to uncover environmentally susceptible astrocyte pathways (Fig. 1a). We utilize these screens in combination with genome-wide transcriptome profiling such as scRNA-seq (Fig. 1b) and other tools of our own design to understand how astrocytes can be controlled to modulate the pathogenesis of neurologic diseases, such as multiple sclerosis.
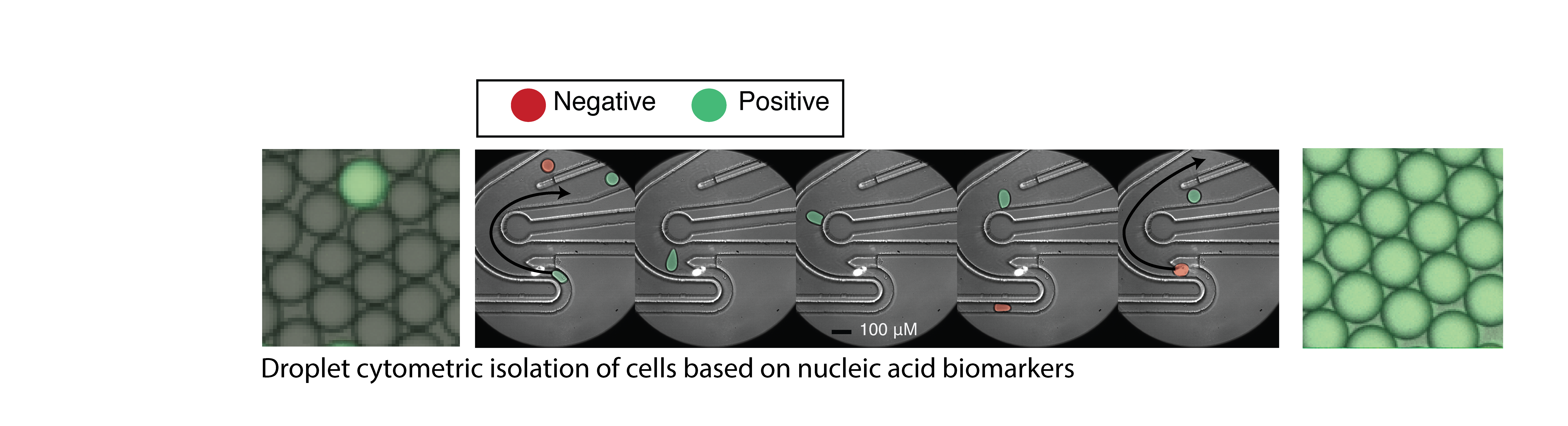
High throughput droplet microfluidics for single cell analysis. Crosstalk between cells of different lineages is a hallmark of nervous system function in health and disease, but tools to study cell communication are limited by throughput and ease of use. We are developing new technologies to study cellular interactions in the CNS at single cell resolution. These new methods will enable direct investigation of the specific cell types, pathways, and molecules that mediate pro- and anti-inflammatory cell networks in the context of CNS inflammation and neurodegeneration.
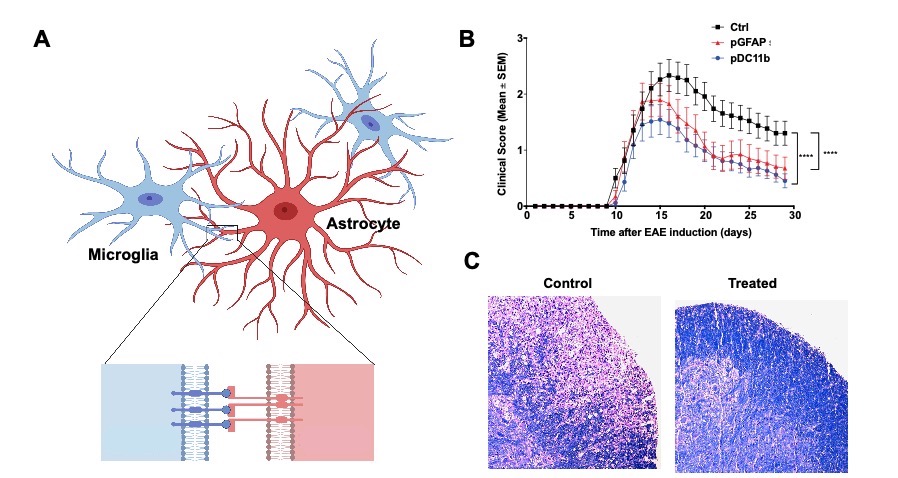
Fascinated by the crosstalk between immune cells and very interested in the functional interaction between CNS resident cells in the context of neuroinflammation. In one of the projects, we have uncovered a novel pathway that mediates microglia and astrocytes crosstalk promoting their pathogenic activities during EAE, Eph receptor / signaling (Figure 1A). When we disrupted the pathway we were able to ameliorate the disease (Figure 1B and 1C) unraveling a promising target for therapeutic intervention of neuroinflammatory diseases.
Figure 1 Reciprocal control of microglia and astrocytes via signaling
The interaction of the immune system with the environment has crucial implications in the context of autoimmunity that can be repurposed for the treatment of autoimmune diseases like multiple sclerosis and inflammatory bowel disease (IBD). Thus, we have been using engineered commensal microorganisms in order to target pathogenic pathways to treat these diseases.
One of these projects aimed to activate aryl hydrocarbon receptor (AhR), a tolerogenic transcription factor that is normally activated by small molecules provided by the diet and the gut flora. Indeed, using conditional knockout mice, transgenic mice expressing traceable proteins and single cell RNA seq, we demonstrated that EAE is ameliorated when mice are fed with novel bacteria engineered to produce AhR agonists and we are currently addressing the mechanisms of this findings. This project was developed in close collaboration with SYNLOGIC company.
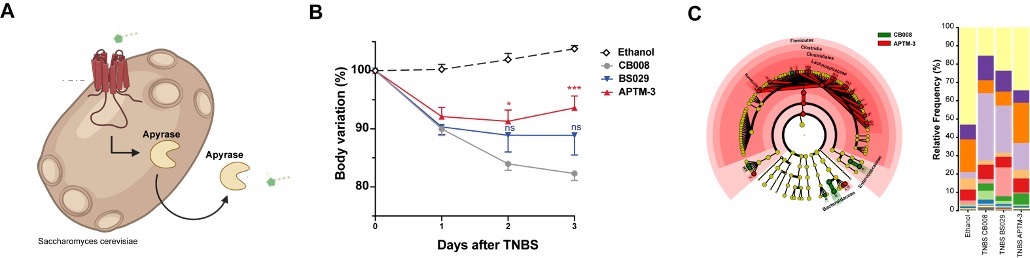
Additionally, in a different project, we have targeted extracellular adenosine triphosphate known to be associated to intestinal inflammation and IBD pathology. We created synthetic yeast probiotics capable of sensing and at the same time producing apyrase in a proportional self-regulated fashion to degrade (Figure 2A). Treatment with these yeasts ameliorated experimental model of IBD (Figure 2B) and restored the gut microbiome (Figure 2C).
Figure 2 Self-Tunable Engineered Anti-Inflammatory Yeast Probiotics